Dec 16, 2020 - Health
Extra doses of Pfizer vaccine could expand U.S. supply
Add Axios as your preferred source to
see more of our stories on Google.
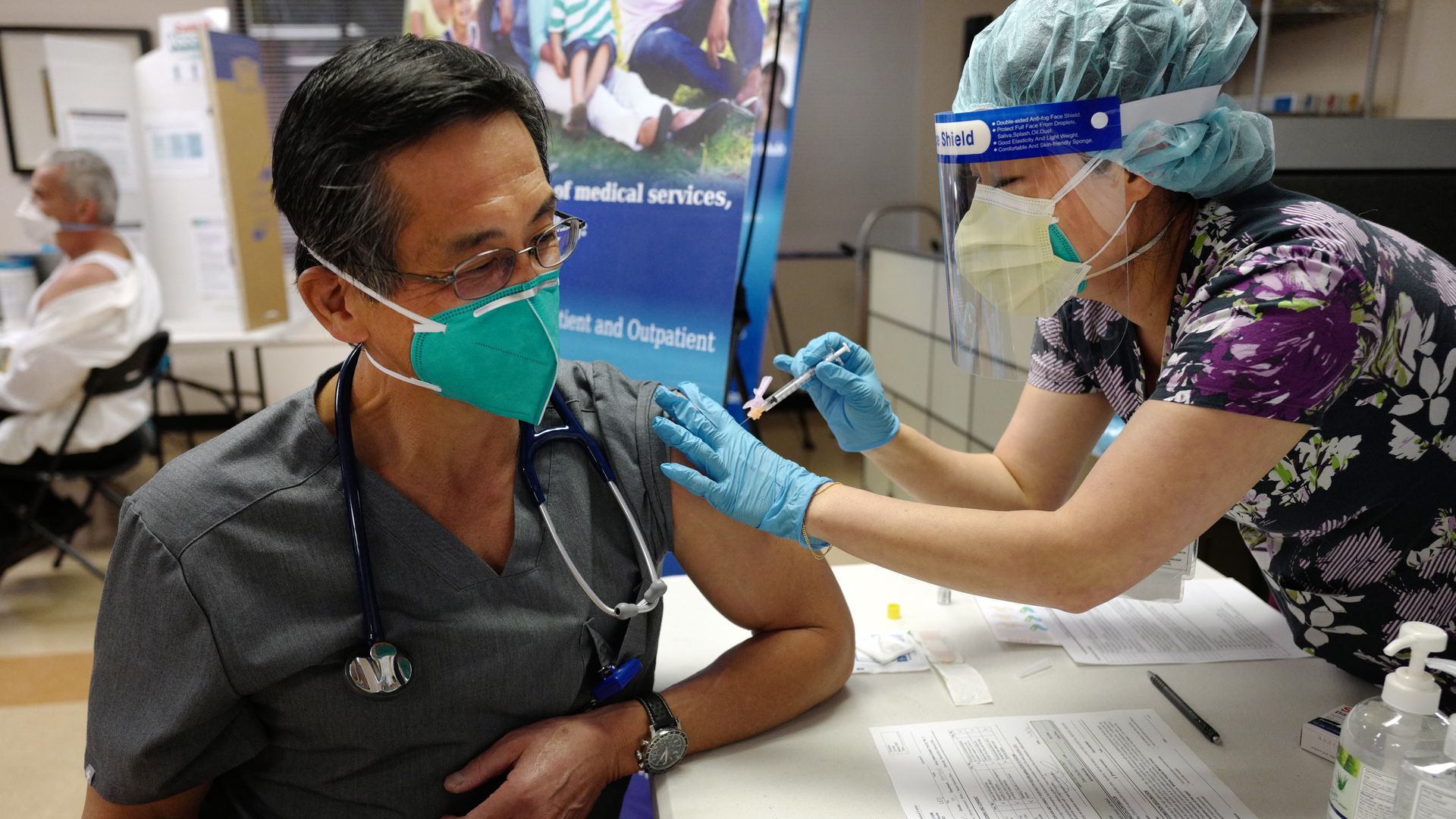
An infectious disease doctor in California receives the Pfizer vaccine on Thursday, Dec. 17. Photo: Keith Birmingham/MediaNews Group/Pasadena Star-News via Getty
