Jan 25, 2024 - Business
Robitussin cough syrup recalled for microbial contamination
Add Axios as your preferred source to
see more of our stories on Google.
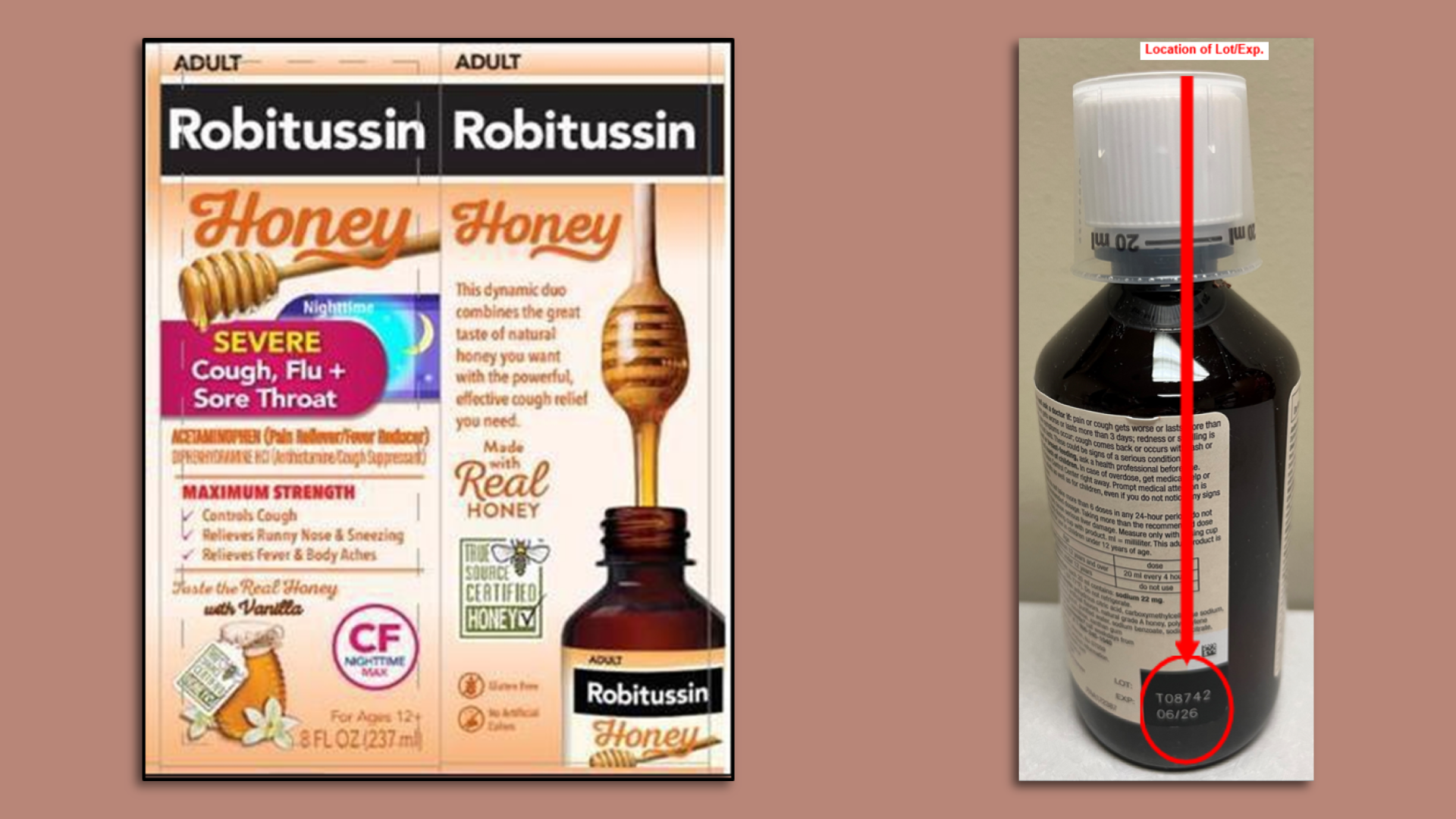
Select lots of Robitussin Honey cough syrups have been recalled. Photos: Courtesy of Haleon via FDA website
