Dec 8, 2020 - Health
FDA review of Pfizer vaccine clears way for emergency authorization
Add Axios as your preferred source to
see more of our stories on Google.
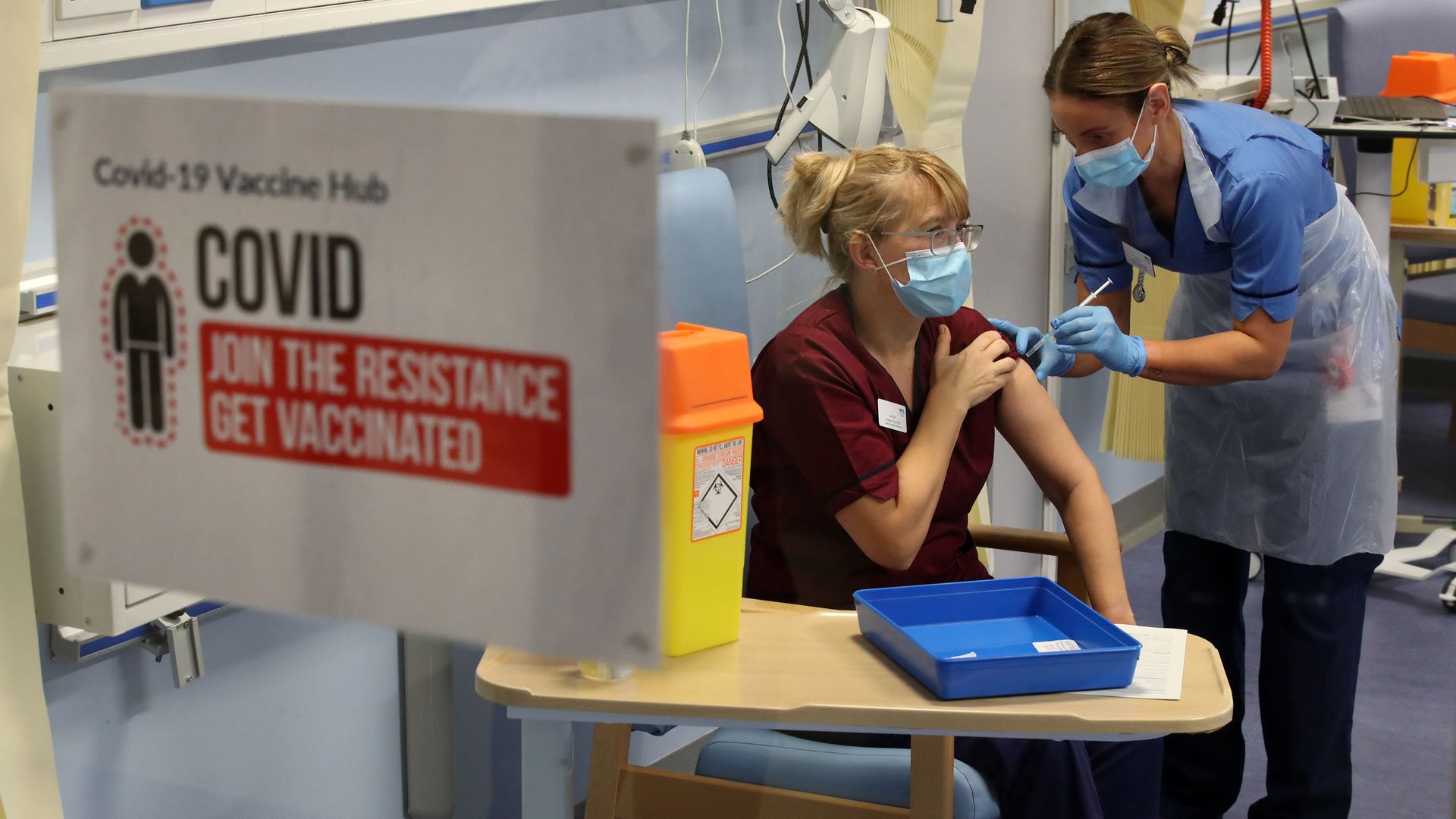
Deputy charge nurse Katie McIntosh administers the first of two Pfizer/BioNTech COVID-19 vaccine jabs to clinical nurse manager Fiona Churchill in Edinburgh, Scotland on Dec. 8. Photo: Andrew Milligan/pool/AFP via Getty Images
