Aug 26, 2020 - Health
FDA authorizes Abbott's $5 rapid COVID-19 test
Add Axios as your preferred source to
see more of our stories on Google.
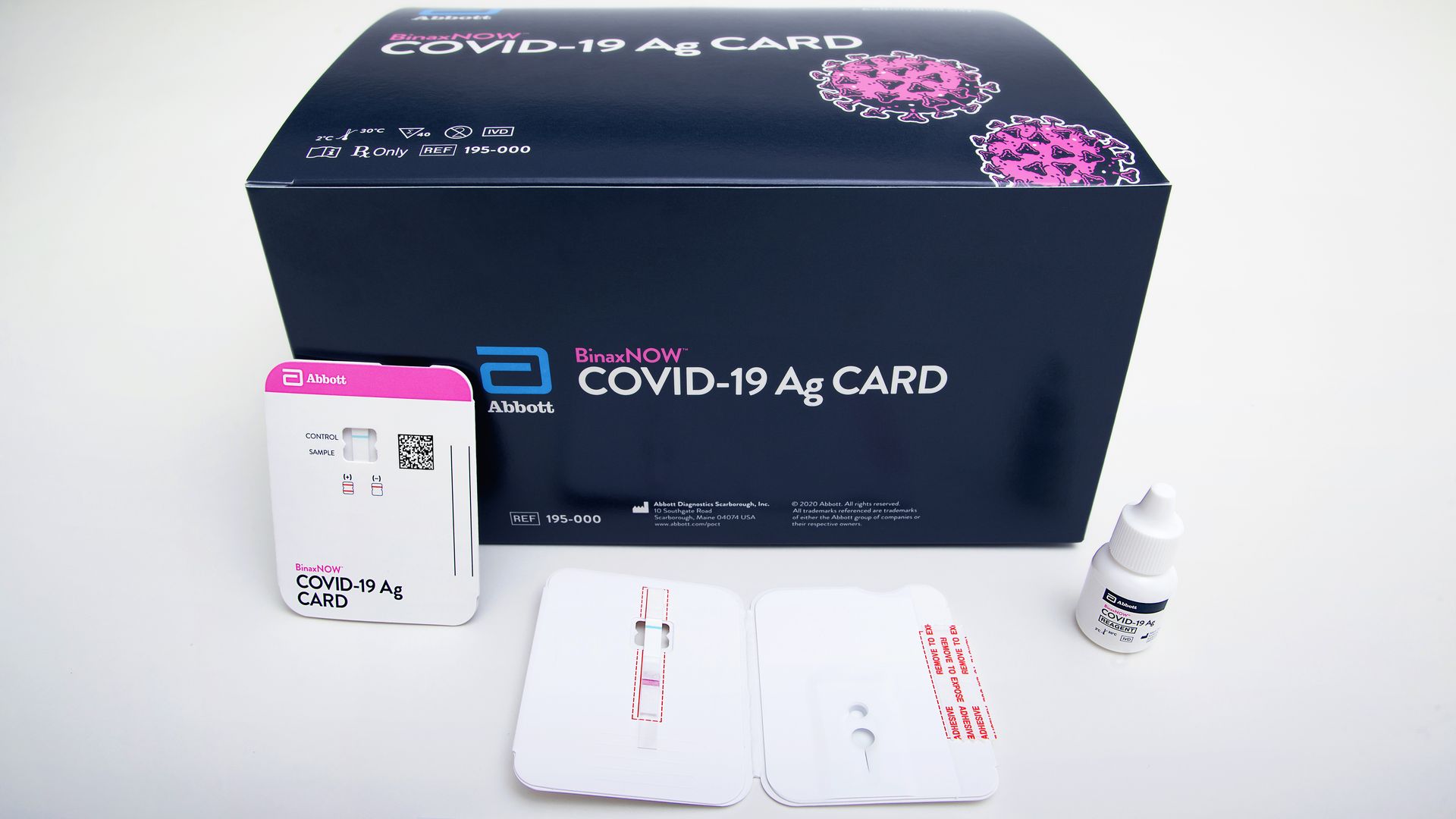
Results from the BinaxNOW COVID-19 Ag Card test will be available in roughly 15 minutes. Photo: Courtesy of Abbott Laboratories.
