Apr 26, 2022 - Health
Pfizer asks FDA to authorize COVID booster for children 5 to 11
Add Axios as your preferred source to
see more of our stories on Google.
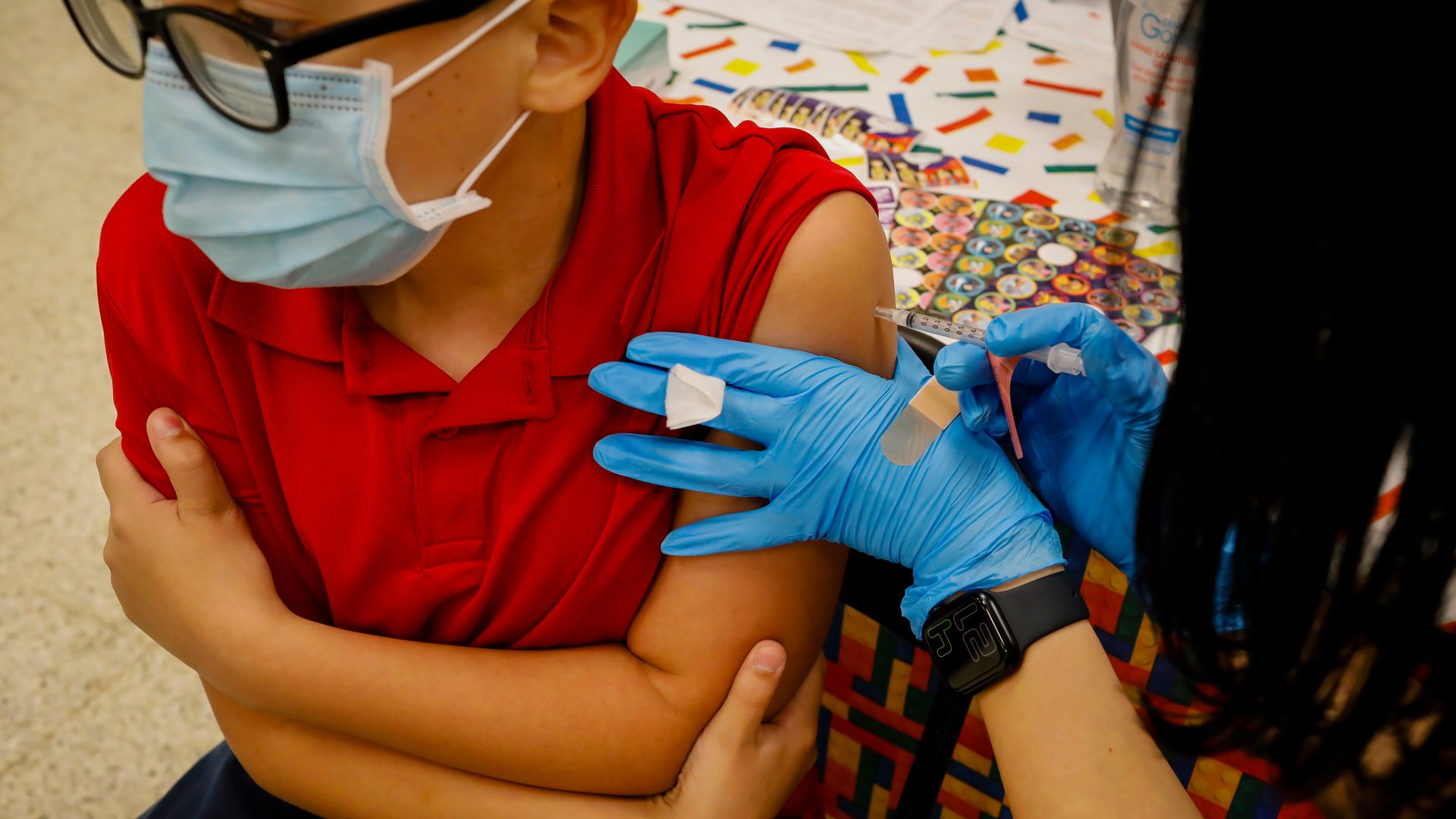
A child receives a dose of the Pfizer-BioNTech COVID-19 vaccine at an elementary school vaccination site for children ages 5 to 11 in Miami on Nov. 22, 2021. Photo: Eva Marie Uzcategui/Bloomberg via Getty Images
