Nov 4, 2021 - Health
WHO approves Indian COVID vaccine in boost for global supply efforts
Add Axios as your preferred source to
see more of our stories on Google.
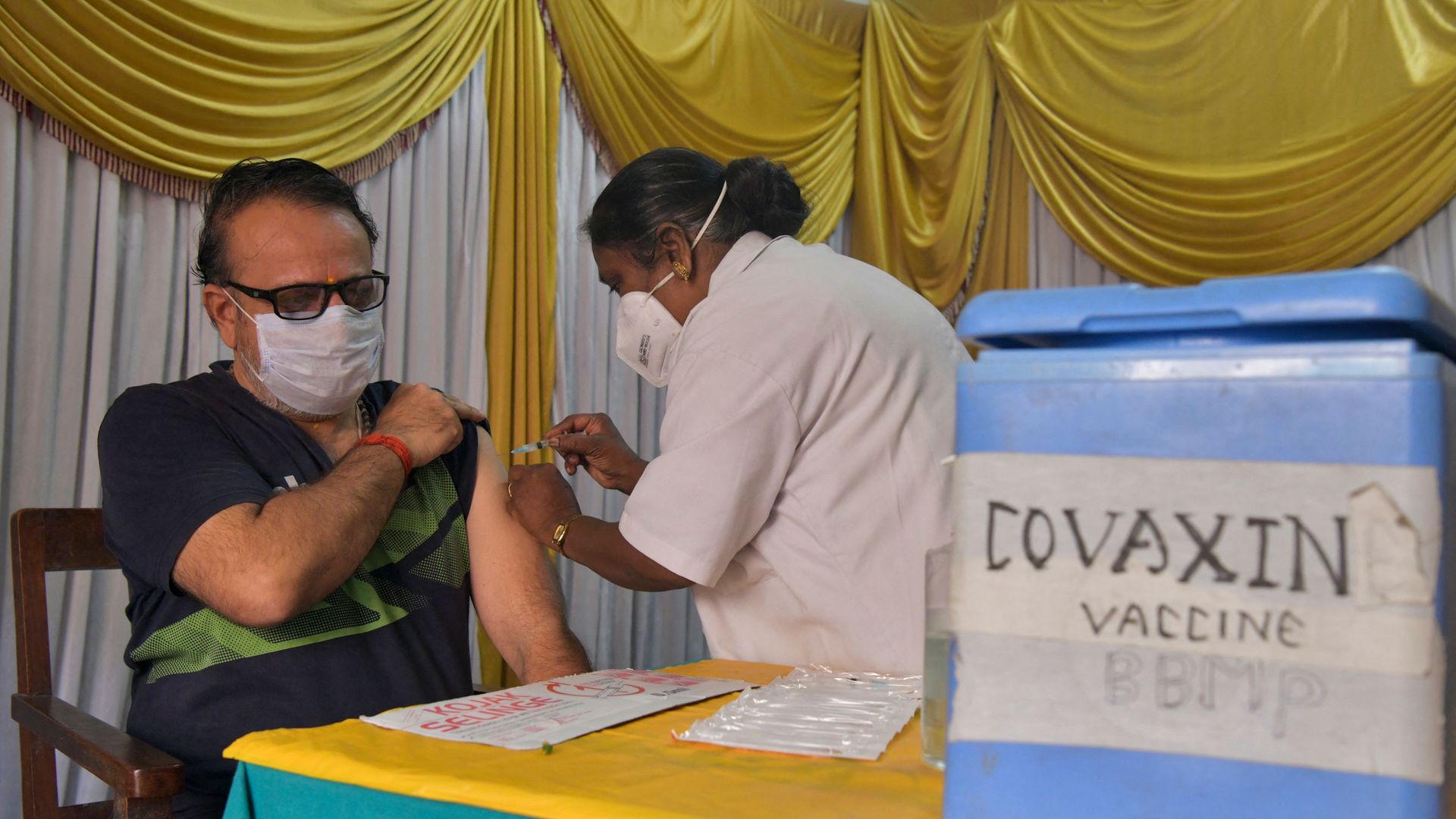
A health worker inoculates a man with a dose of Covaxin’s COVID-19 vaccine in Bangalore, India, in June. Photo: Manjunath Kiran/AFP via Getty Images
