Jun 1, 2021 - Health
Moderna applies for full FDA approval of COVID-19 vaccine
Add Axios as your preferred source to
see more of our stories on Google.
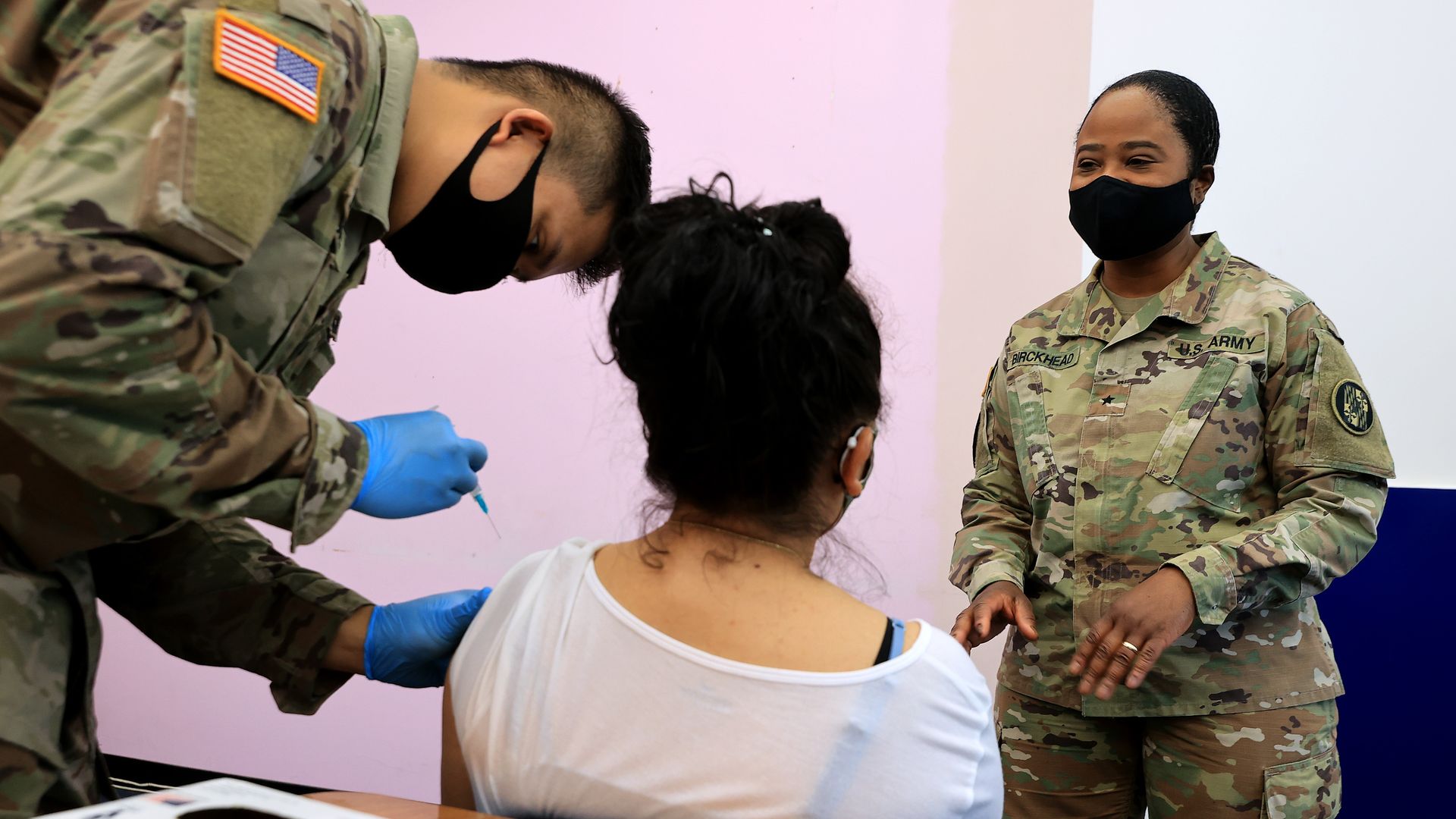
Maryland National Guard Brigadier General Janeen Birckhead visits with a woman as she receives her Moderna coronavirus vaccine from Specialist James Truong (L) on May 21 in Wheaton, Maryland. Photo: Chip Somodevilla/Getty Images
