Jan 21, 2022 - Health
FDA OKs antiviral drug remdesivir for non-hospitalized COVID patients
Add Axios as your preferred source to
see more of our stories on Google.
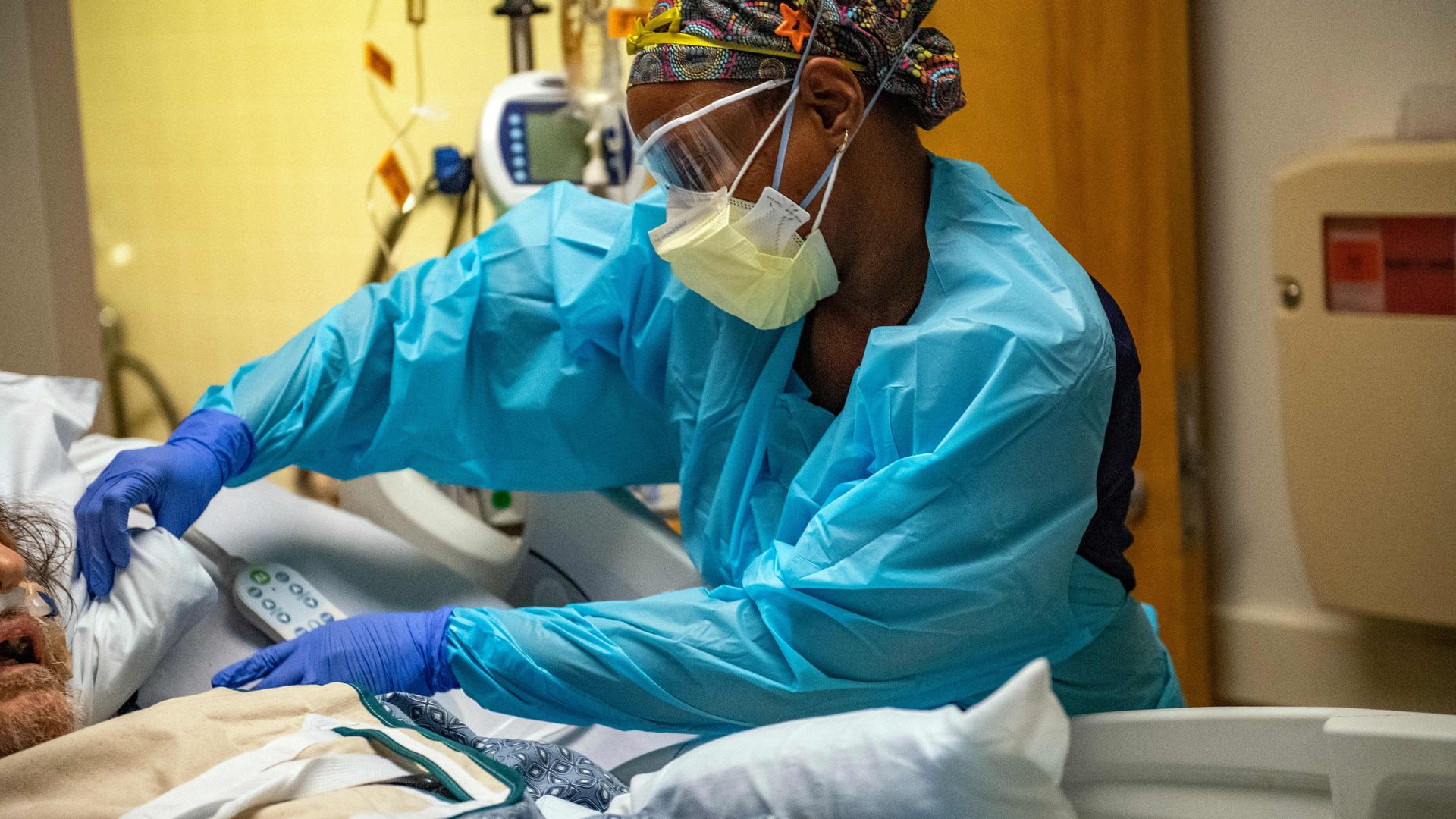
A medical worker in full PPE works on a patient who has COVID in a negative pressure room in the ICU ward at UMass Memorial Medical Center in Worcester, Massachusetts, on Jan. 4. Photo: Joseph Prezioso/AFP via Getty Images
