Updated Nov 19, 2021 - Health
CDC recommends Pfizer, Moderna COVID boosters for all adults
Add Axios as your preferred source to
see more of our stories on Google.
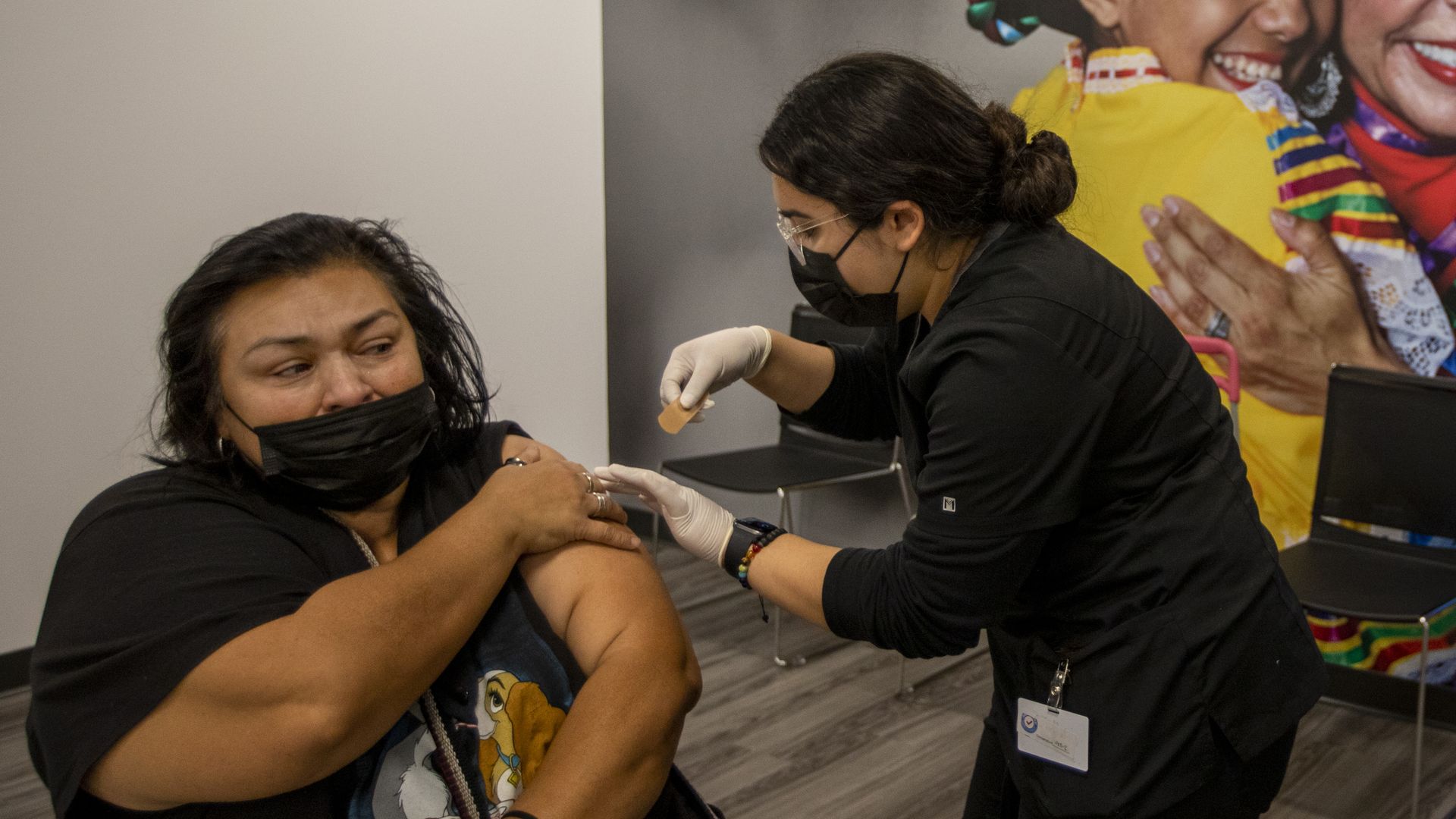
Irene Michel, right, gives Santana Ruiz a COVID-19 vaccination in El Monte, California, on Nov. 17. Photo: Francine Orr/Los Angeles Times via Getty Images
