Oct 29, 2021 - Health
FDA authorizes Pfizer vaccine for kids 5 to 11
Add Axios as your preferred source to
see more of our stories on Google.
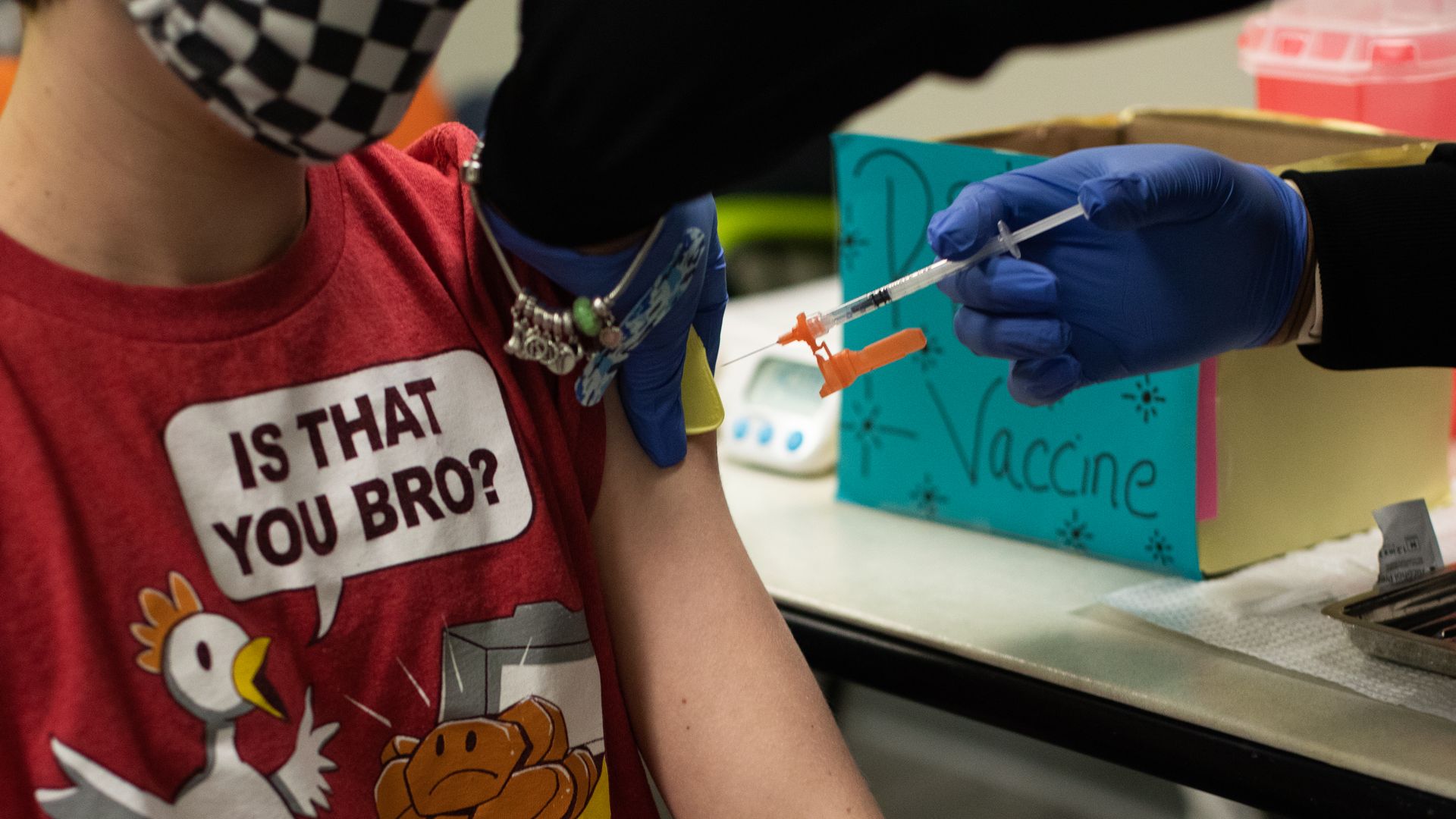
A health care worker administering a dose of Pfizer-BioNTech's Covid-19 vaccine to a child in Bingham Farms, Michigan, in May 2021. Photo: Emily Elconin/Bloomberg via Getty Images
