Feb 6, 2021 - Health
China approves Sinovac vaccine, its second shot for general public use
Add Axios as your preferred source to
see more of our stories on Google.
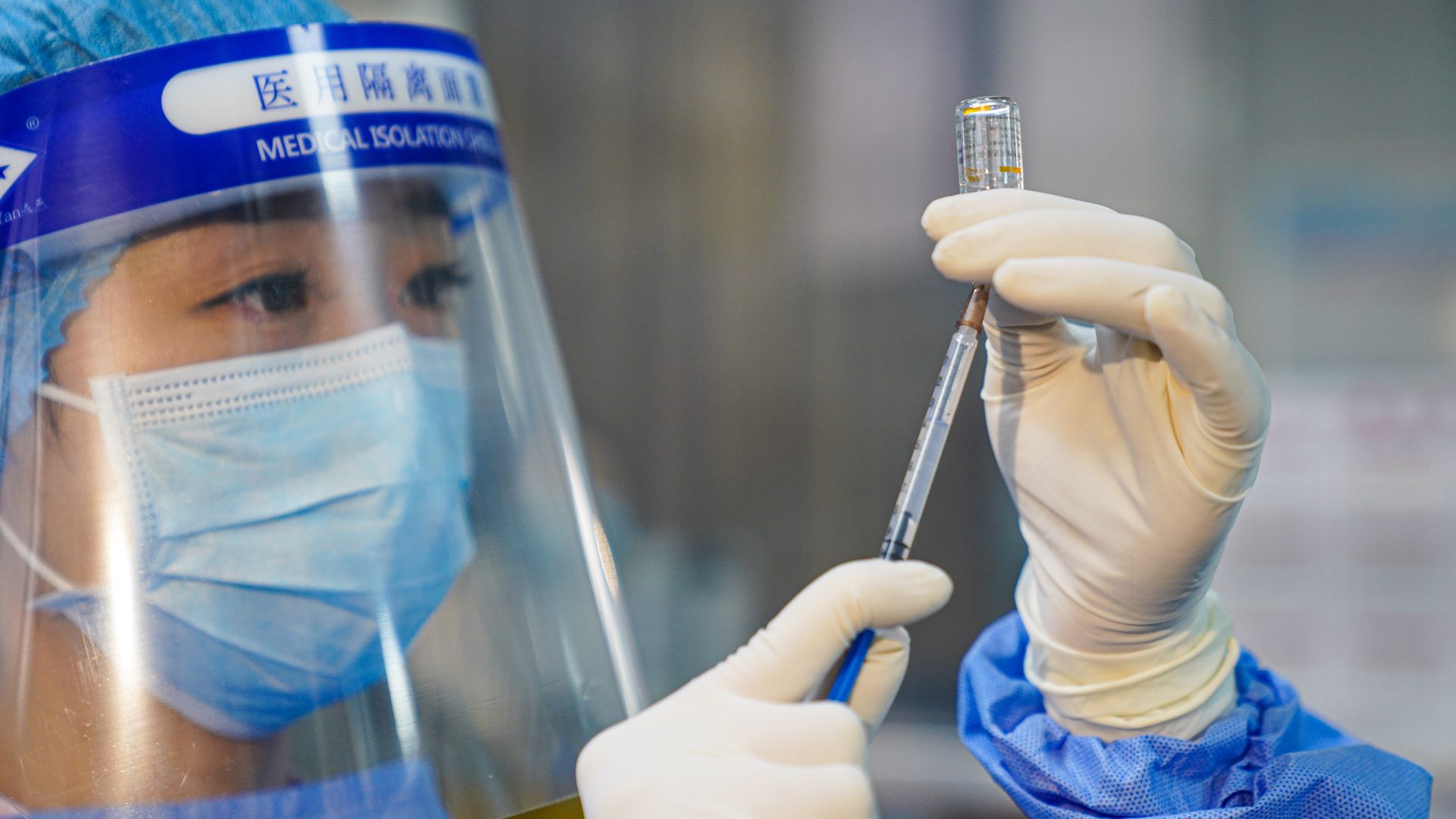
A nurse prepares a Sinovac's COVID-19 vaccine in China. Photo: VCG/VCG via Getty Images
