Sep 30, 2020 - Health
Moderna says its coronavirus vaccine won't be ready until 2021
Add Axios as your preferred source to
see more of our stories on Google.
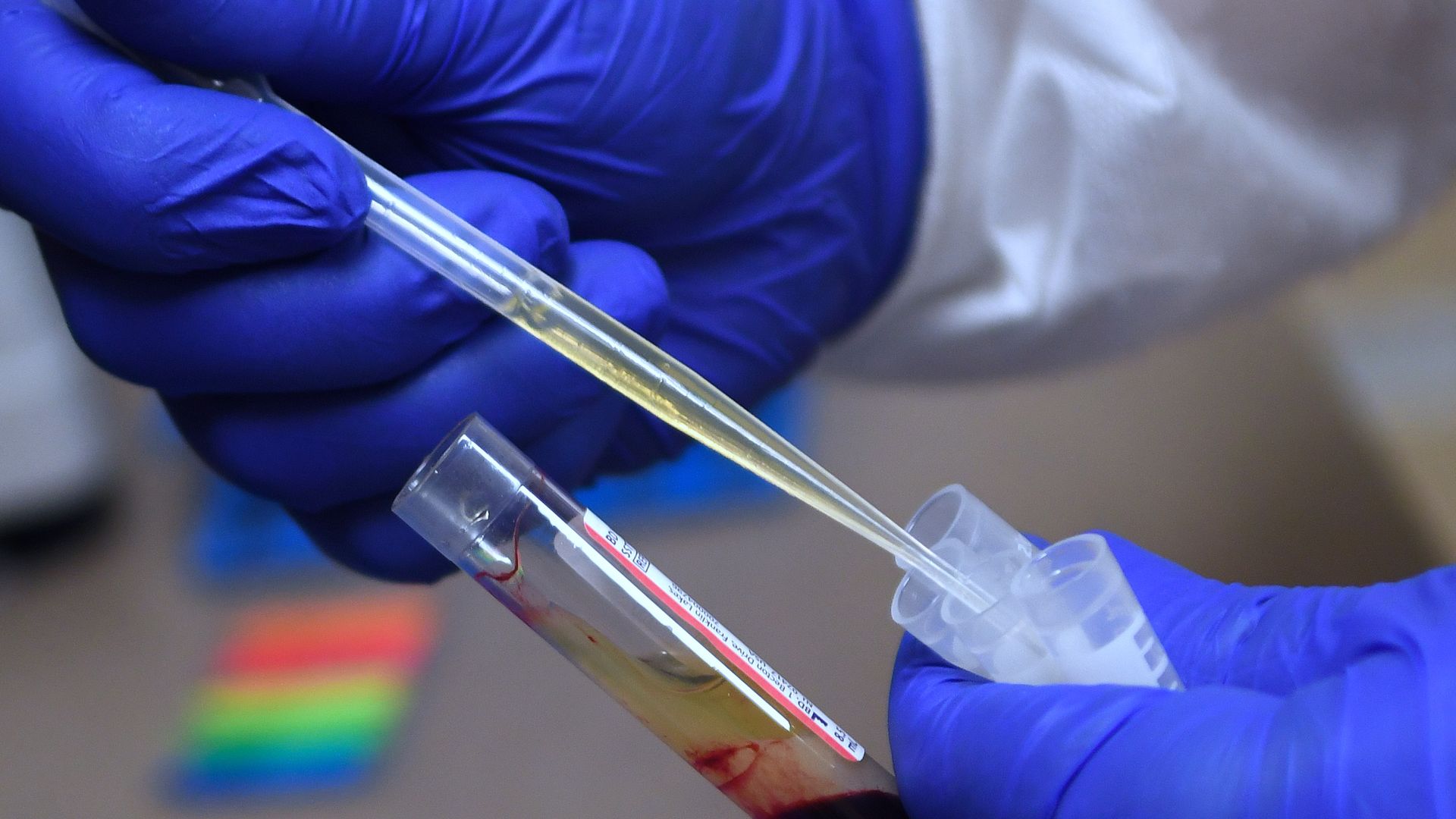
A laboratory technician preparing a blood sample for a vaccine clinical trial sponsored by Moderna. Photo: Paul Hennessy/NurPhoto via Getty Images
