May 16, 2020 - Health
FDA authorizes first at-home kit that can be used with multiple coronavirus tests
Add Axios as your preferred source to
see more of our stories on Google.
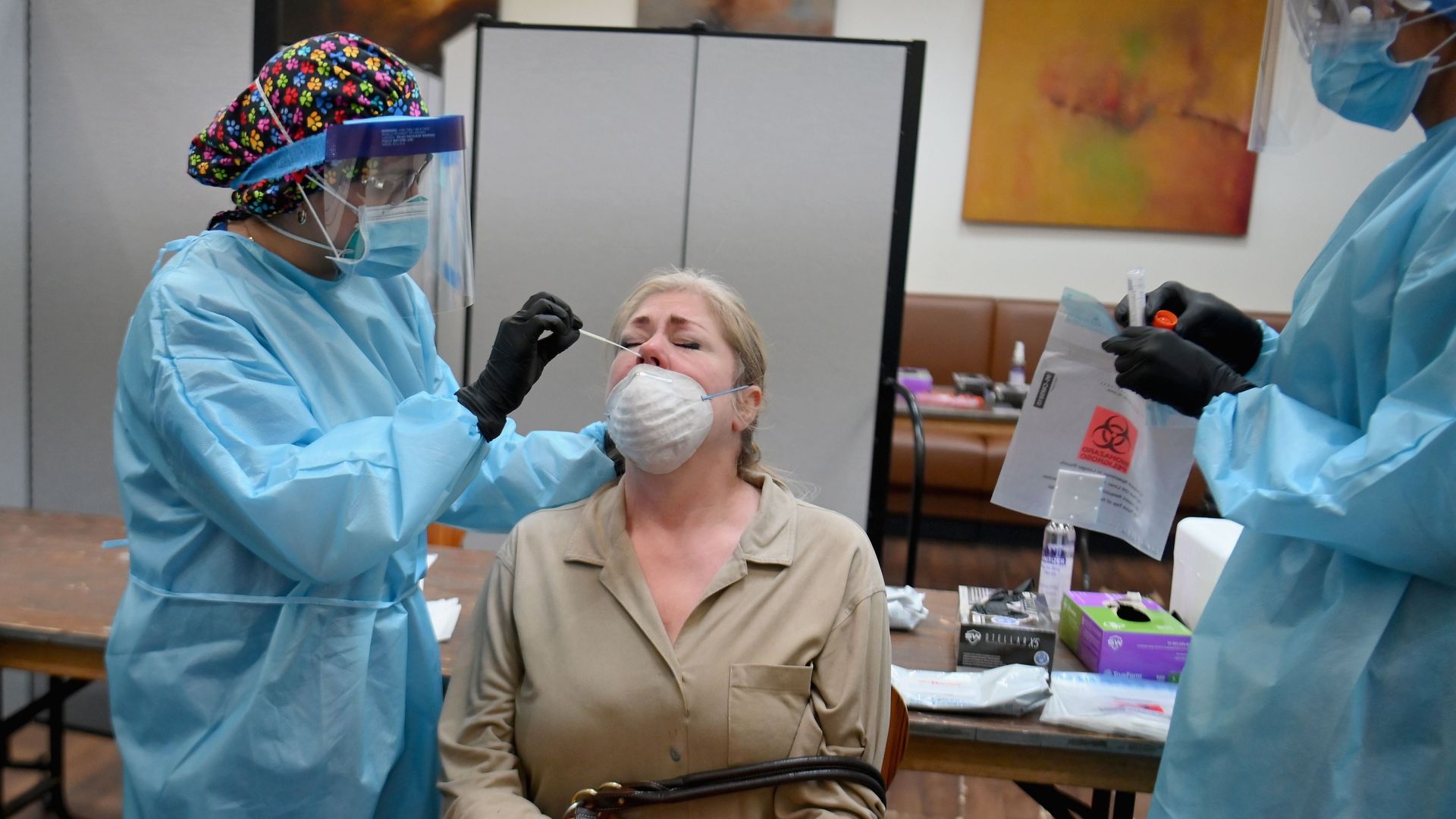
A health care worker takes a nasal swab sample to test for the coronavirus at the Abyssinian Baptist Church in Harlem, New York City on May 13. Photo: Angela Weiss/AFP via Getty Images
