Oct 21, 2019
FDA approves drug therapy that would treat 90% of people with cystic fibrosis
Add Axios as your preferred source to
see more of our stories on Google.
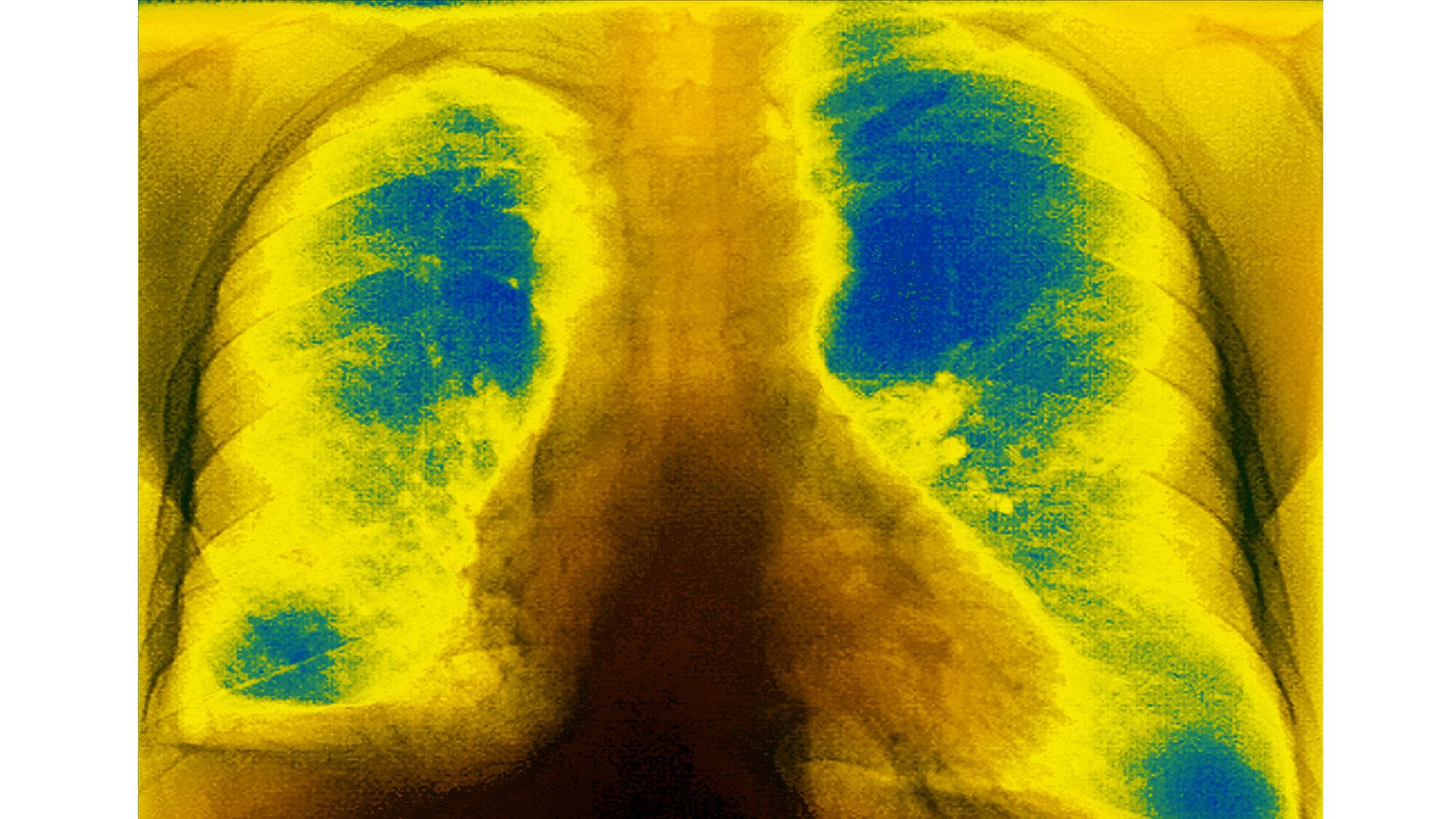
Mucoviscidosis (accumulation of mucus in the respiratory tract), seen on a frontal X-ray of the chest. Photo: BSIP/Universal Images Group via Getty Images
